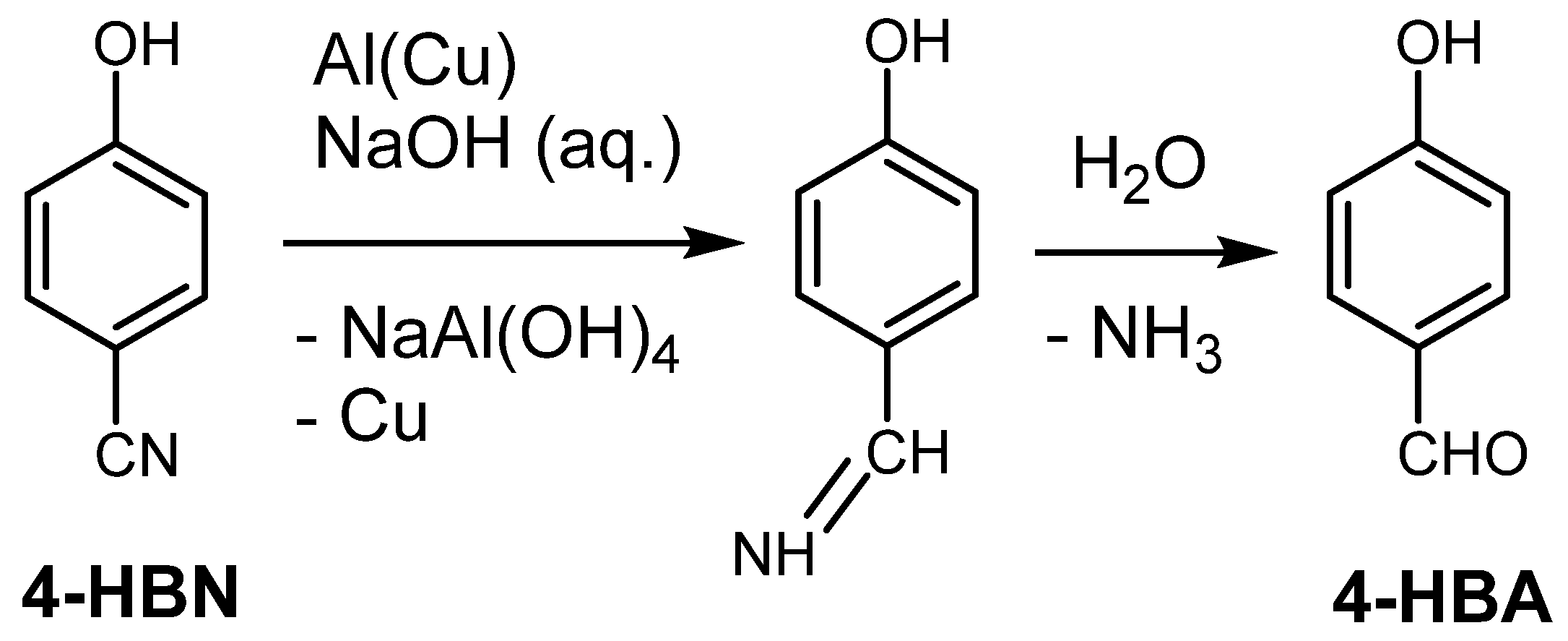
Catalysts | Free Full-Text | Cu-Catalyzed Hydrodehalogenation of Brominated Aromatic Pollutants in Aqueous Solution

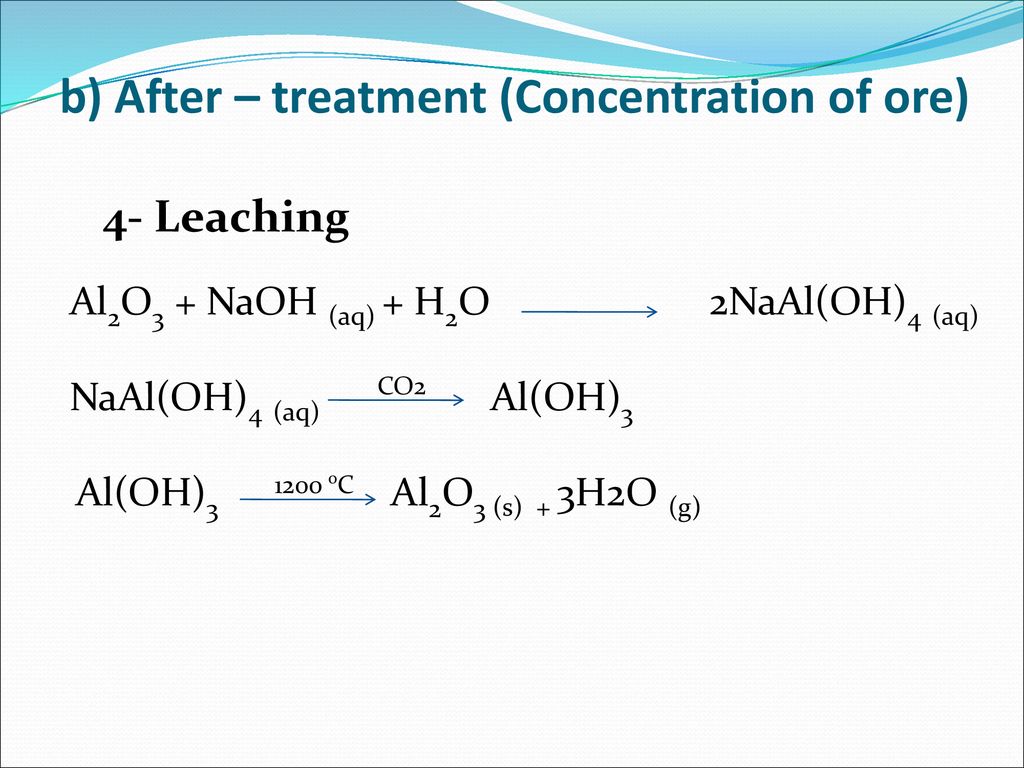
Setarakan persamaan reaksi redoks menggunakan cara bilangan oksidasi atau ion elektron Al + NaOh + H2O - Brainly.co.id
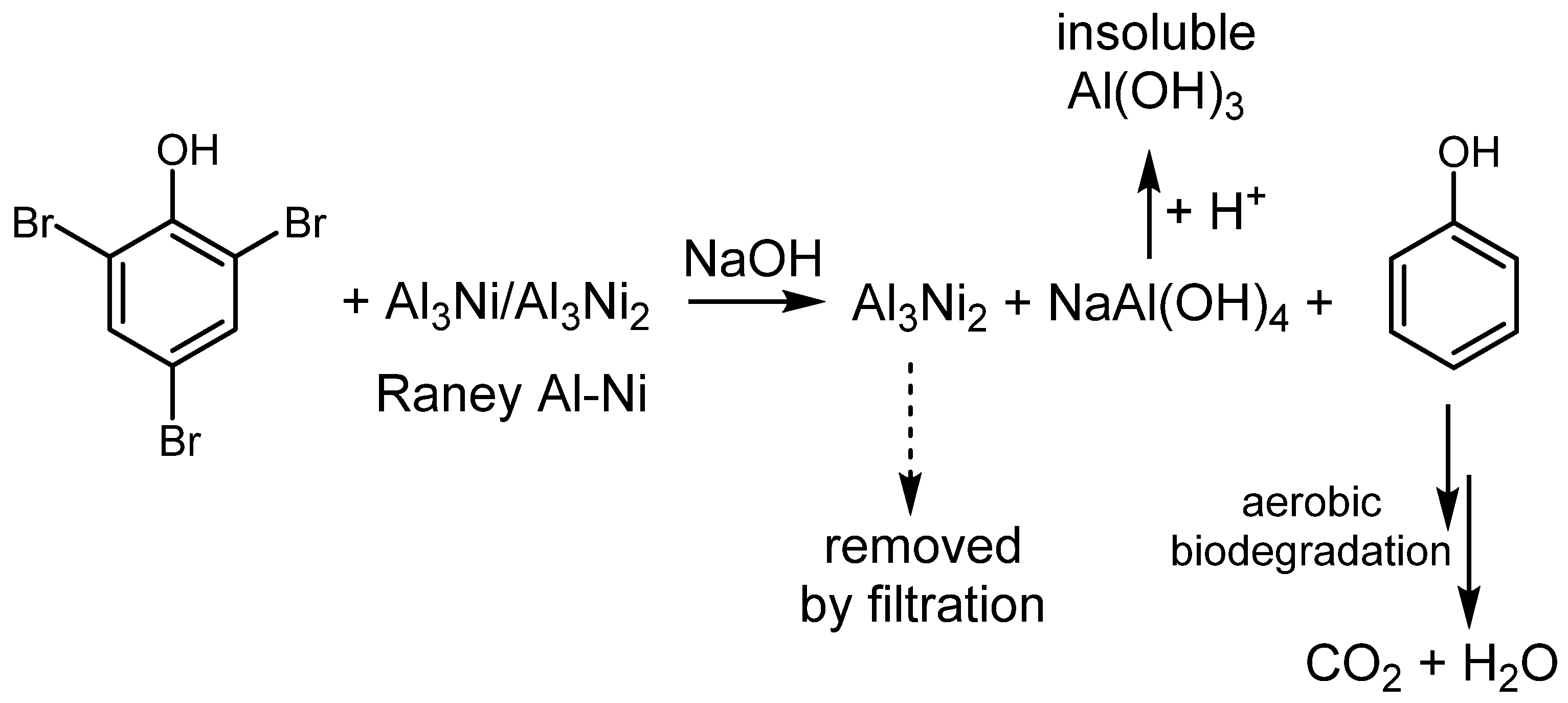
Catalysts | Free Full-Text | Applicability of Nickel-Based Catalytic Systems for Hydrodehalogenation of Recalcitrant Halogenated Aromatic Compounds

Separation of NaOH and NaAl(OH)4 in alumina alkaline solution through diffusion dialysis and electrodialysis - ScienceDirect

Dissolution and Absorption: A Molecular Mechanism of Mesopore Formation in Alkaline Treatment of Zeolite | Chemistry of Materials
![PDF] Theoretical studies on aluminate and sodium aluminate species in models for aqueous solution: Al(OH)3, Al(OH)-4, and NaAl(OH)4 | Semantic Scholar PDF] Theoretical studies on aluminate and sodium aluminate species in models for aqueous solution: Al(OH)3, Al(OH)-4, and NaAl(OH)4 | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6a77c7699e5a02a7ca2fbc332c05618dd4a3a75a/4-Figure1-1.png)
PDF] Theoretical studies on aluminate and sodium aluminate species in models for aqueous solution: Al(OH)3, Al(OH)-4, and NaAl(OH)4 | Semantic Scholar

Solubility and Modeling of Sodium Aluminosilicate in NaOH–NaAl(OH)4 Solutions and Its Application to Desilication | Industrial & Engineering Chemistry Research
![PDF] Theoretical studies on aluminate and sodium aluminate species in models for aqueous solution: Al(OH)3, Al(OH)-4, and NaAl(OH)4 | Semantic Scholar PDF] Theoretical studies on aluminate and sodium aluminate species in models for aqueous solution: Al(OH)3, Al(OH)-4, and NaAl(OH)4 | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6a77c7699e5a02a7ca2fbc332c05618dd4a3a75a/8-Table6-1.png)
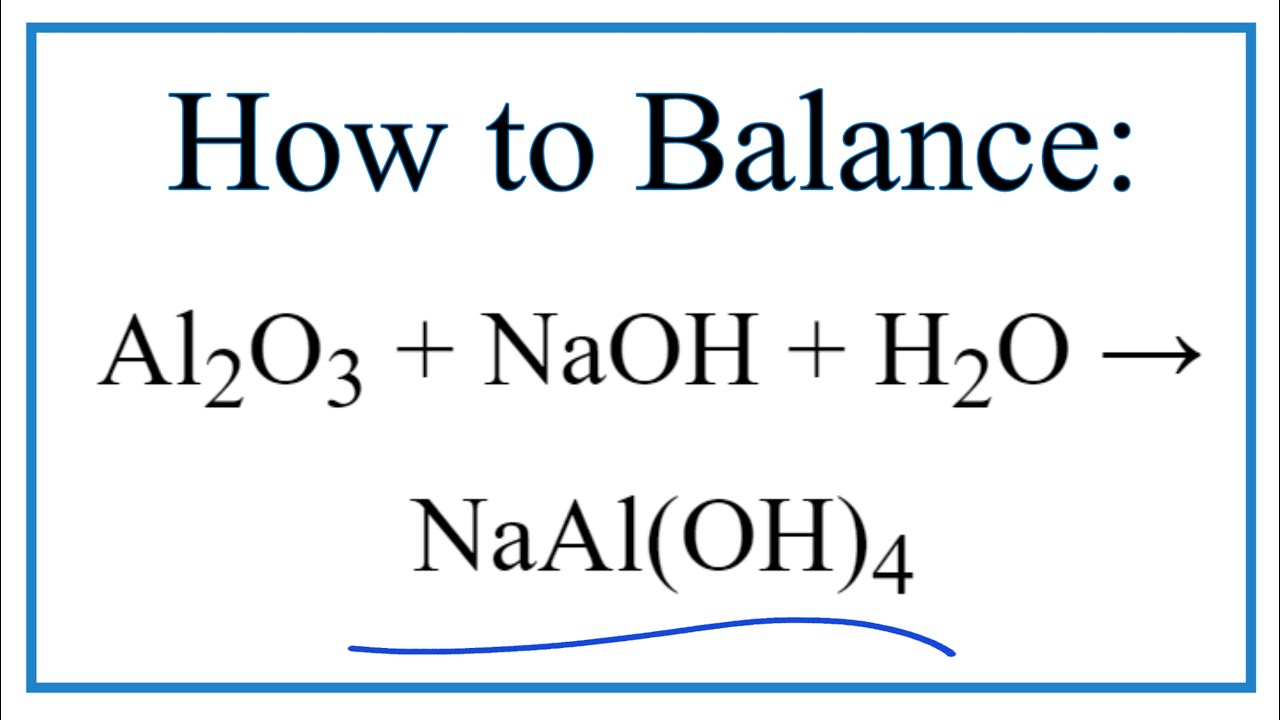


![Al(OH)3 + NaOH → Na[Al(OH)4] | Aluminium hydroxide dissolving in an excess of alkali - YouTube Al(OH)3 + NaOH → Na[Al(OH)4] | Aluminium hydroxide dissolving in an excess of alkali - YouTube](https://i.ytimg.com/vi/_u6eAdWg96A/maxresdefault.jpg)

![Al + NaOH + H2O = Na[Al(OH)4] + H2 | Aluminum react with sodium hydroxide and water Al + NaOH + H2O = Na[Al(OH)4] + H2 | Aluminum react with sodium hydroxide and water](https://i.ytimg.com/vi/IP7RBIJL724/maxresdefault.jpg)






![Al + NaOH = Na[Al(OH)4] + H2 : r/chemistry Al + NaOH = Na[Al(OH)4] + H2 : r/chemistry](https://external-preview.redd.it/AeR6aLSSGt_SU77x2CsbCPHsT_VAHbl_fOQfQVQQaPQ.png?format=pjpg&auto=webp&s=3044c582b9874a0918a7ff51cc6db7f5292db280)
